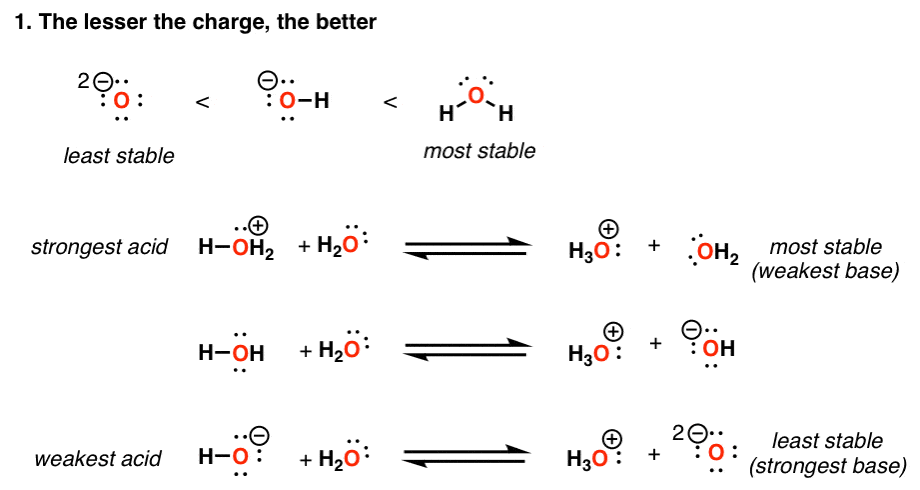
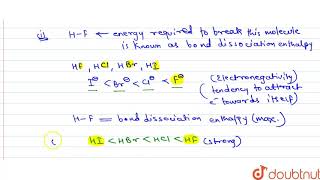
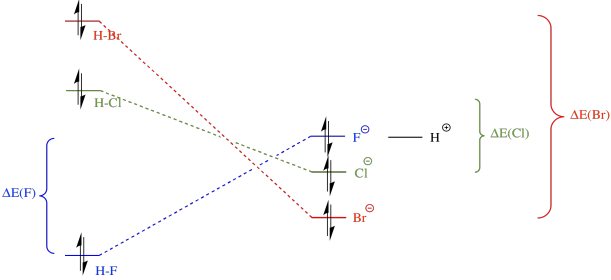
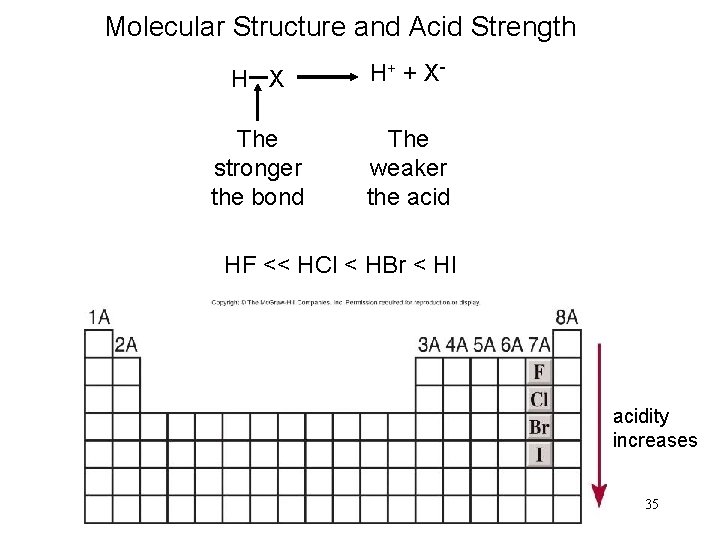
Acidity increases in the order HF, HCl, HBr, HI Note that in water dissociation is fairly complete Acidity may be related to the reaction HA rightleftharpoonsH^ A^ The stronger the acid;Si Consider the acids, H, H2S, H2SeDue to the high electronegativity of flourine hydrogen bonds can be formed between HF molecules Hydrogen bonds require more energy to break that London Forces The other halogens are not as electronegative and so other hydrogen halides cannot form hydrogen bonds between moleculesOnly London Forces are formed Therefore more energy is required to break the intermolecular forces in HF
How Does Electronegativity Affect The Strength Of An Acid Socratic
Hf vs hi acidity
Hf vs hi acidity- HCl is a stronger acid than HF because fluorine is more electronegative than chlorine I'm thinking this is true, because since fluorine is more electronegative than chlorine, it has a higher ionic energy and therefore a stronger bond between hydrogen than chlorine does with hydrogen In result, leading HCl to dissociate more easily Can you please clarify acidbase Share ImproveAcidity means ability to readily give H ions The order of the most acidic will be HI > HBr > HCl > HF Since H is a small atom, and I is a big atom, so it would be easy for them to break the bond, hence H ions are most readily given While H and F are small atom, so they have a strong bond between them (high bond enthalpy) and so, they would be least acidic



Solved 17 Which Of The Following Compounds Is Most Acidic Chegg Com
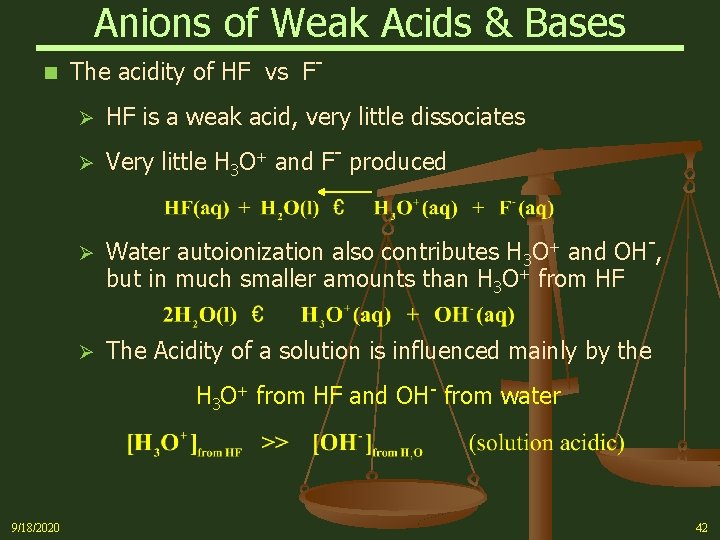
Acid HA AKa pKa Acid Strength Conjugate Base Strength Hydroiodic HI IHydrobromic HBr BrPerchloric HClO4 ClO4Hydrochloric HCl ClChloric HClO3 ClO3Sulfuric (1) H2SO4 HSO4Nitric HNO3 NO3Strong acids completely dissociate in aq solution (Ka > 1, pKa < 1) Conjugate bases of strong acids are ineffective bases Hydronium ion H3O H2O 1 00 Iodic HIO3 IO316 x 101PH Vs Hydrofluoric Acid Concentration 3 32 34 36 38 4 42 44 46 0 5 10 15 25 30 35 40 45 50 Weight Percent HF pH All statements and information are believed to be accurate and reliable, they are presented without guarantee or warranty of any kind, express or implied Information provided herein does not relieve the user from the responsibility of carrying out its Hydrofluoric acid or HF is an extremely corrosive acid However, it is a weak acid and not a strong acid because it does not completely dissociate in water (which is the definition of a strong acid) or at least because the ions it forms upon dissociation are too strongly bound to each other for it to act as a strong acid
The concentration of hydrofluoric acid (HF) is calculated from conductivity and temperature readings Valmet provides ready made recipes for measuring the hydrofluoric acid concentration or the amount of water in anhydrous hydrofluoric acid All equipment is delivered readily calibrated so there is no need for hazardous sampling on site All you have to do is install the sensor andHF, or Hydrofluoric Acid, is a highvolume chemical that is extremely corrosive It is miscible with water with the release of heat and an acrid, irritating odor, forming a clear, colorless liquid HF is a significant health hazard as a liquid, even in diluted quantities HF vapor, or Hydrogen Fluoride gas, can cause severe injury through contact, inhalation or ingestion Free fluoride ionsDear learner Welcome to decision in this session We are going to identify the correct statement about the assets from the given options in this problem So w
Trend HF, Ha, HBr, HI Rank the halogens in order of increasing electronegativity Rank the halogens in order of increasing size Circle the acids that are on your strong acid list IIF IICI HBr HI Rank the acids in order of increasing strength Which characteristic (size or electronegativity) is more important in explaining the strength of these acids? Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity Because of the fluoride ion's small size, it cannot disperse the negative charge over a larger space and will have an extremely high affinity for an electrophile (like $\ce{H}$), and because of this it will remain mostly as $\ce{HF}$Contact us on below numbers For Study plan details / 1000 AM to 700 PM IST all days For Franchisee




Relative Order Of Acid Strength Of Halogen Acids Hx In Aqueous Solution Hf Hcl Hbr Hi Pdf Youtube



Acidity Trends In Organic Chemistry Master Organic Chemistry
Re HF, HCl, HBr, and HI Acid Strength Postby Julia Kim 1D » Sun 1047 pm It's because HF has the strongest bond that it makes it the weakest out of all of them The stronger the acids, the more easily it dissociates in solution Since HF has the strongest bond, it doesn't dissociate as easily as HI does, making it weaker HI is a stronger acid than HF since iodine is less electornegative than fluorine Thus, the H proton is less attracted to the iodide anion and more willing to be donatedAcidity constants are taken from here ) The limited solubility of hydroxides is taken into account (as indicated by footnotes in the last column) Hydroxides are strong bases but have low




Descriptors For The Hydrogen Halides Their Solution Properties And Hydrogen Bonding Acidity And Basicity Comparison Of The Latter With Gas Phase Data Sciencedirect



3 4 Structural Effects On Acidity And Basicity Organic Chemistry
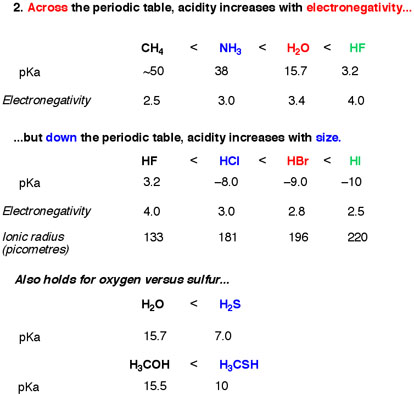
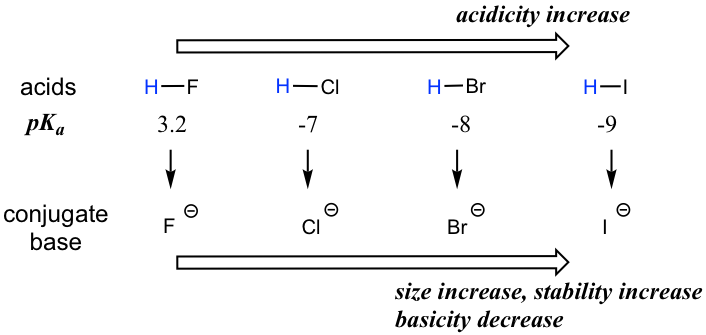
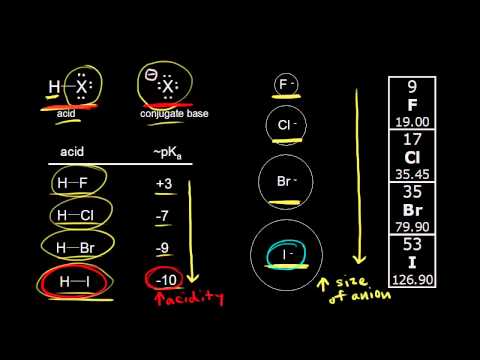
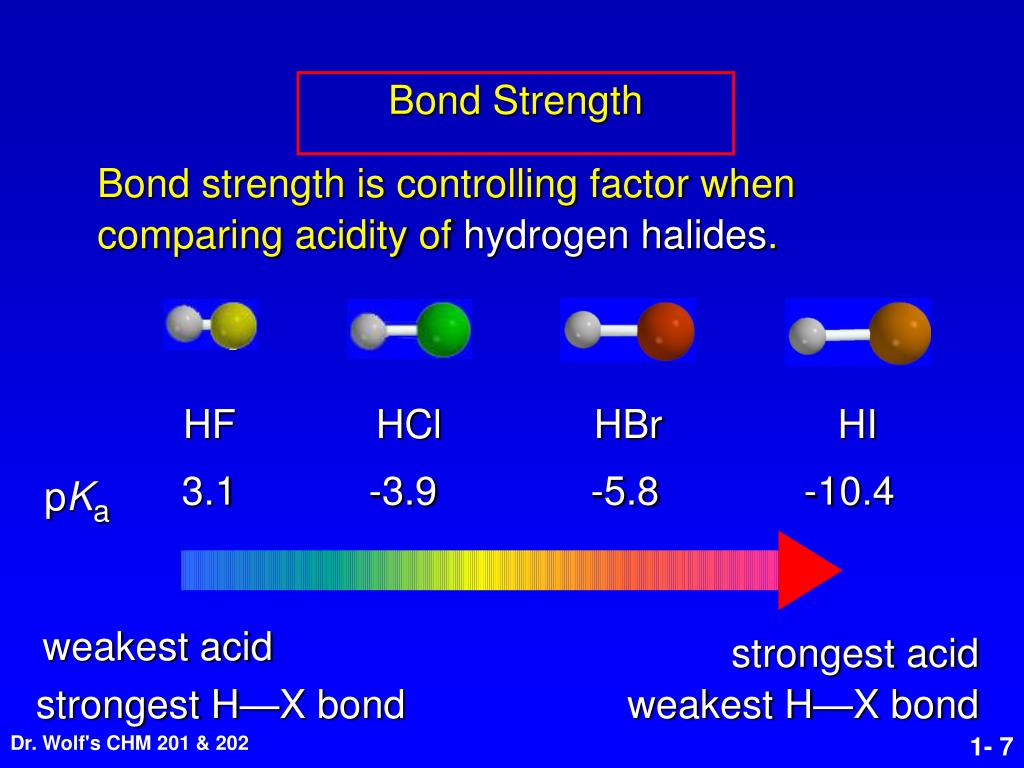
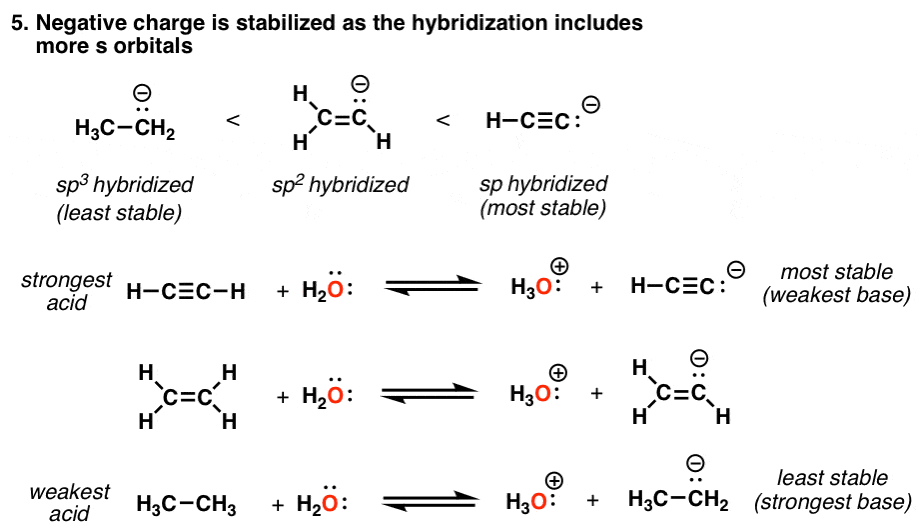
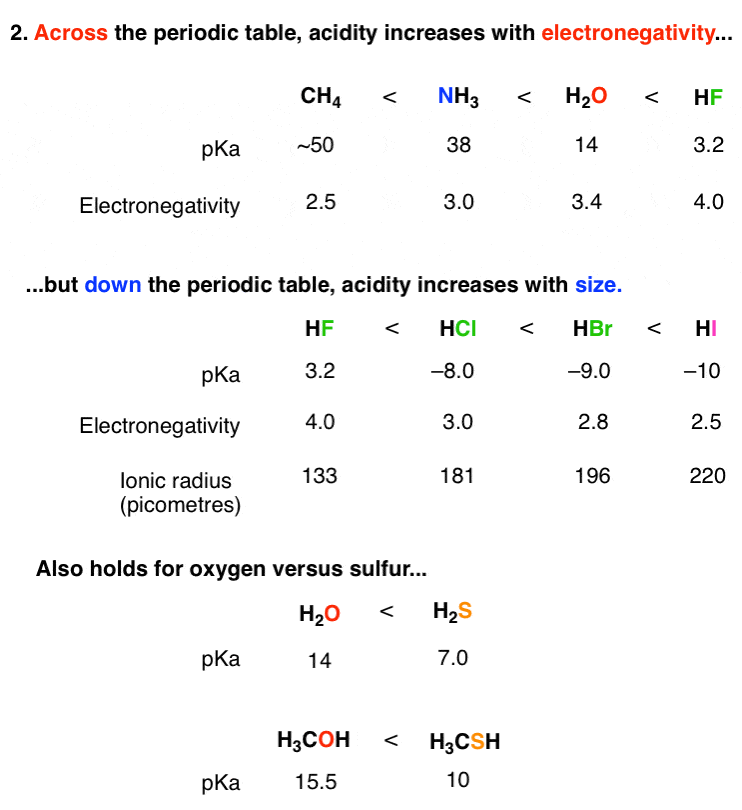
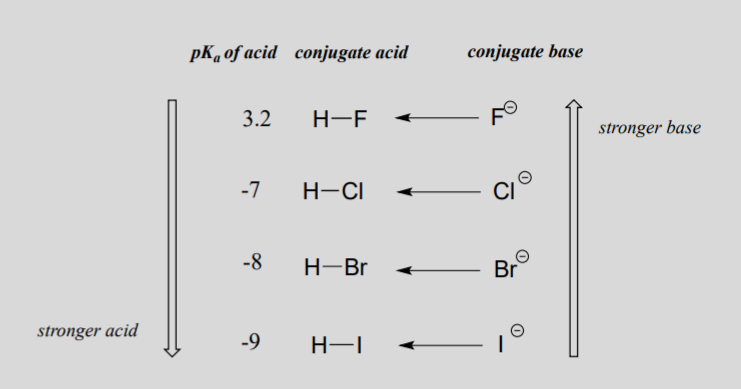
The acidity of the series of hydrohalic acids, HF, HCl, HBr and HI increases from HF through HI, a fact that seems counterintuitive given that fluorine is the most electronegative and iodine the least of the four halides Several factors come into play including heat of formation of the hydrohalides (ΔH f o), bond dissociation energy (BDE), electron affinity (EA) and heat ofBecause HF isthe weakest acid, F isthe strongest base b Because HI isthe strongest acid, I isthe weakest base 16 Chapter 2 13 a oxygen As you saw in Problem 11, the size of an atom is more important than its electronegativity in determining stability So even though oxygen ismore electronegative than sulfur, H 2S is a stronger acid than H, and CH3SH is a stronger acid thanHCl, HBr, and HI are all strong acids, whereas HF is a weak acid The acid strength increases as the experimental pKa values decrease in the following order HF (pKa = 31) < HCl (pKa = 60) < HBr (pKa = 90) < HI (pKa = 95) Hydrochloric acid Hydrochloric acid is a clear, colorless solution of hydrogen chloride (HCl) in water It is a highly corrosive, strong mineral acid with many



Organic Acids And Bases




Learn Chemistry Periodicity In Properties Facebook
Hydrofluoric Acid HF With a pKa of 315 HF is considered a weak acid in that it is not full disassociated below a pH of about 55 and will form complexes with many compounds including itself Do not let the classification as a weak acid fool you, HF is a very aggressive and dangerous acid HF is commercially available in concentrations ranging from 10% to 49% with 49% HF beingPost by Chem_Mod » Wed 242 am Question Why does the bond strength decrease in the following order HF (strongest)> HCl bond> HBr > HI bond?HF is a weaker acid because the strength of an acid is determined by how completely that acid will dissociate Since the bond




15 Which Of The Following Order Si Not Correct A Boiling Point Hf Hci Hbr Hi
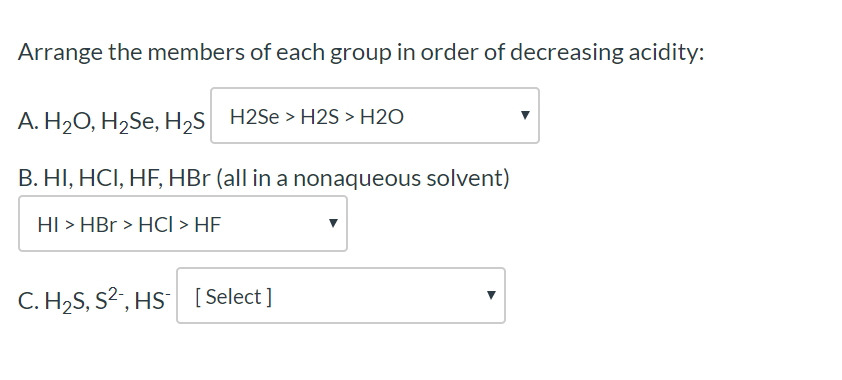



Solved Arrange The Members Of Each Group In Order Of Chegg Com
Get answer Consider the following orders (i) HF > HCl > HBr > HI Acidic strength (ii) CH_2 lt "CCl"_4 gt CF_4 Electronegativity of central 'C' atom (iii) Mg^(2) lt K^() lt S^(2) lt Se^(2) Ionic radius (iv) Ni > Pd > Pt Ionic radius (v) As^(5) gt Sb^(5) gt BI^(5) Stable oxidation state (vi) Li^() lt Mg^(2) lt Al^(3) Hydration energy (vii) Cl > Br > F > IHI or H2Te comparing strongest acid, so since HI is only one H attached to iodine (binary) don't i need to compare with atomic sizes I vs Te?HF in ammonia is more acidic than HF in water, leveling effect, effect of solvent in acidity have been explained by chemistryconcept faculty Mr Gaurav Jhaa




Hi Is Stronger Than Hf In Acidic Strength Why




Acid Base Oxide
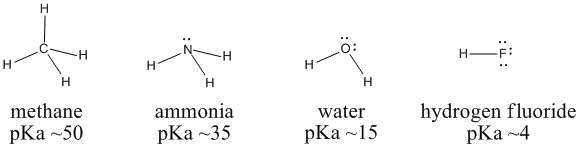
pH of Common Acids and Bases Calculated pH values of common acids and bases for 1, 10, and 100 mmol/L (valid for standard conditions at 25, 1 atm; ch4 nh3 h2o hf this is the increasing order of acidity give reason Chemistry TopperLearningcom OneonOne Online Live Interactive Doubt Solving Classes Book a session today × Contact Us Contact Need assistance?0 votes 1 answer (a) Assign reasons for the following (i) The acidic strengths of acids increases in the order HF < HCl< HBr < HI asked in Chemistry by Nisa (598k points) cbse;
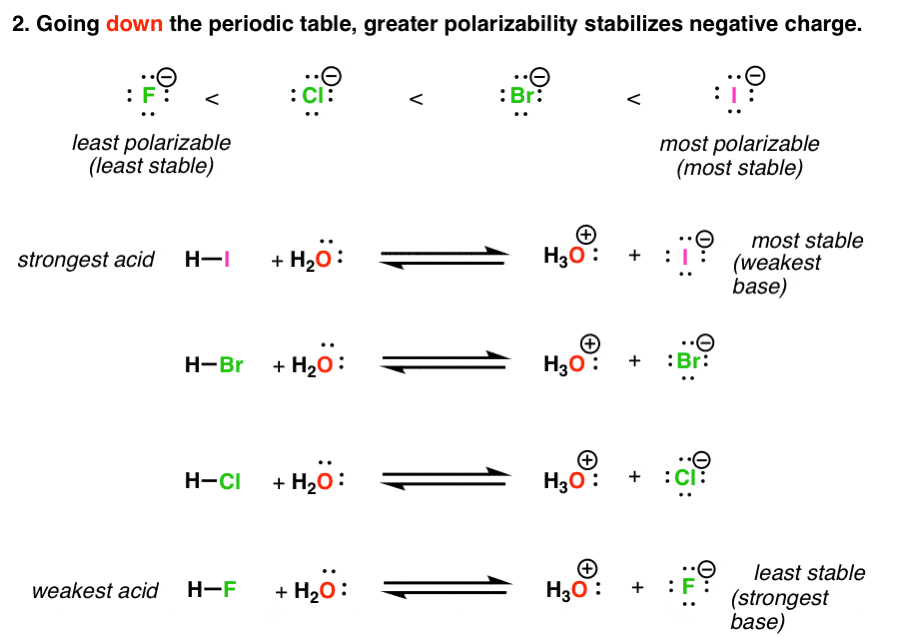



Acidity Trends In Organic Chemistry Master Organic Chemistry



Hydrohalic Acids
HF is a weak acid, but very dangerous (you may hear it etches glass, that's because the SiF bond is the strongest bond) HCl and the rest of them (HBr, HI) are all "strong acids" but HI is the strongest (see pKa's below) Due to poor orbital overlap (Iodine is much larger than H, and therefore the electrons are not very well shared, which imparts an extremely ionic character to HF > HCl > HBr > HI? HI is a stronger acid than HF since iodine is less electornegative than fluorine Thus, the H proton is less attracted to the iodide anion and more willing to be donated




Arrange The Following Acids In The Decreasing Order Of Their Acid Strength Hf Hcl Hbr Hi Youtube




Vertical Pore Water Profiles Of Sulfate Fe And Acidity At The End Of Download Scientific Diagram
Of high selectivity – then use this material as the mask • SiO 2 Wet etching of silicon dioxide is accomplished in a dilute solution of Hydrofluoric Acid (HF)dilute solution of Hydrofluoric Acid (HF) • Dilutions in water of H 2 OHF61, 101 & 1 are commonly used • Etch rate of thermal oxide in 61 HF solution is about about 10Å/min Lecture 19 EE 441 Spring 09The further to the right lies this equilibrium Of course, the acidity is modified by the identity of the solvent In water, the acid base reaction is often represented as HX(g) H_2O(lHydrogen iodide ( H I) is a diatomic molecule and hydrogen halide Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas They are interconvertible




Acids Bases Part Ii Strong Vs Weak Acids




Fb35jlhhr9bypm
Acidic vs Alkaline Foods So how exactly is the list of acidic and alkaline food groups determined?Produces concentrated hydrofluoric acid (HF), which has strong corrosion capabilities This test was conducted to gain a better understanding of corrosion rates of different highalloy materials in concentrated HF and to help formulate a changeout schedule for CP parts The CP involves many parts and materials of construction, but the parts of most concern are the valves These valvesHydrogen fluoride mixes readily with water forming hydrofluoric acid For all practical purposes, they are considered the same chemical Hydrogen fluoride/hydrofluoric acid is used extensively in the extraction, processing, and refining of metals, rock, brick, and oil It is an intermediate for many chemical reactions and syntheses It is used



Why Hf Is The Weakest Acid Among Hcl Hbr And Hi Though Its Electronegetivity Is Highest Quora




Acids And Bases Properties That Determine Acid Strength Shmoop
Arrange the following acids in the increasing order of their acidic character HF, HCI, HBr and HI asked in Chemistry by Bhawna (685k points) cbse;Why do i have to look at electron negativity which then HI is stronger?Acid Strength of HF, HCl, HBr, and HI Hydracids composed of Hydrogen and Halogens are acidic due to the highly polarised covalent bond between the




The Art Of Writing Reasonable Organic Reaction Mechanisms



Acidity Hf Hcl Hbr Hi
Answer (1 of 5) The strength of the acid INCREASES with the DECREASE of overlap between the halogen atom and the hydrogen atom Hydrogen fluoride is thus the weakest acid, and hydrogen iodide is the STRONGEST acid Note also that an entropy component operates inTop Chem_Mod Posts 039I only compare electro negativity if there is a presence of oxygen no?
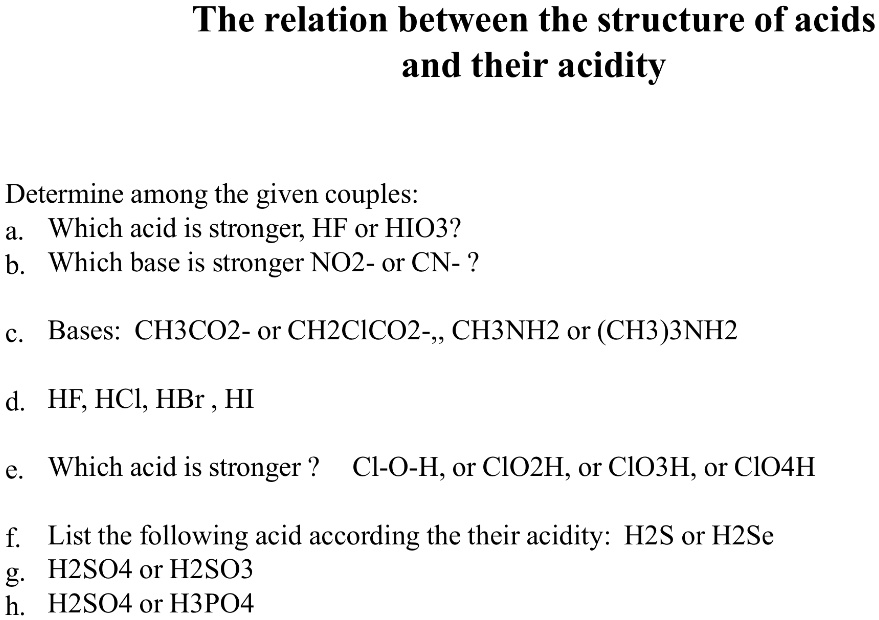



Solved The Relation Between The Structure Of Acids And Their Acidity Determine Among The Given Couples Which Acid Is Stronger Hf Or Hio3 B Which Base Is Stronger No2 Or Cn Bases




Ranking Acidity Organic Chemistry Video Clutch Prep
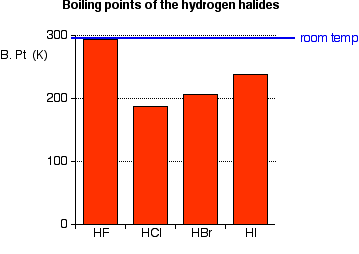
Today South Shore Christian Academy vs South Shore VoTech Massachusetts High School Soccer LIVE HDWatch Here https//vht/vrhpqThe South ShoreAssertion (A) Increasing order of acidity of hydrogen halides is HF lt HCl lt HBr lt HI Reason (R) While comparing acids formed by the elements belonging to the same group of periodic table, HA bond strength is a more important factor in determining acidity of an acid than the polar nature of the bondThe boiling points of HF,HCl,HBr and HI follow the order HF>HI>HBr>HCl HF is hydrogen bonded, thus has highest boiling point, and it is liquid at or below 19 oC The remaining hydrogen halides are gaseous and their boiling points depend on the van der waal's forces Larger the size (or molecular mass), greater are the van der Waal's forces



Ab11 Factors Affecting Bronsted Lowry Acidity



Acid Strength Anion Size And Bond Energy Video Khan Academy
Melting and Boiling points of Hydrogen Fluoride (HF) are higher than Hydrochloric acid (HCl), Hydrobromic acid (HBr) and Hydrogen iodide (HI) Fluorine has the highest electronegativity of an atom When fluorine bonds with hydrogen, the polarity is so strong that it begins to exhibit the property of hydrogen bonding, which is in concentrate just an excessive dipoleHF is a weak acid Medium View solution > HF(mol wt ) boils at high temperature while HCl (mol wt 365) boils at low temperature Easy View solution > The dipole moment of hydrogen halides decreases from HF to HI Explain this trend Medium View solution > View more CLASSES AND TRENDING CHAPTER class 5 The Fish Tale Across the Wall Tenths and Hundredths PartsTo say the same thing in other words It is interesting that over the whole range from p K a = 0 to p K a = 5 or 6, the acid follows the weakacid rule at high concentration, but then follows the strongacid rule at moderately low concentration The meaning of equation 3 is clear it just says that all the acid molecules are ionized (and that autoionization of the water molecules is
:max_bytes(150000):strip_icc()/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png)



List Of Common Strong And Weak Acids



Solved Arrange The Following Acids According To Decreasing Chegg Com
When hydrogen fluoride is dissolved in water, it may be called hydrofluoric acid Hydrogen fluoride can be released when other fluoridecontaining compounds such as ammonium fluoride are combined with water Where hydrogen fluoride is found and how it is used Hydrogen fluoride is used to make refrigerants, herbicides, pharmaceuticals, highoctane gasoline,Hydrogen fluoride is a chemical compound with the chemical formula H F This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid It is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, eg polytetrafluoroethylene (PTFE) HF is widely used in theWhen you eat, the calories and nutrients are extracted from foods, and they are metabolized, leaving behind an ash residue This ash residue is what determines the pH of your food, separating it into either an acidforming or alkalinizing food Acidforming foods typically




How Does Electronegativity Affect The Strength Of An Acid Socratic



Ppt 1 14 What Happened To P K B Powerpoint Presentation Free Download Id
In direct contrast with "HCl" vs "HBr", "HClO" is a stronger acid than "HBrO", because "Cl" is more electronegative, which dominates over the size difference between "Cl" and "Br" due to the presence of the oxygen Therefore, the "H""Cl" bond is weakened more from more uneven sharing of electrons, and weaker bond = stronger acidityHBr, HCl, HF, HI 8 List by formula the following substances in order of increasing boiling point methoxymethane (dimethyl ether), CH 3OCH 3 1,2ethanediol, OHCH 2CH 2OH ethanol, CH 3CH 2OH ethane, CH 3CH 3 Intermolecular Forces Answers 1 All group 17 elements (the halogens) have the same valence electron configuration and exhibit the same type of bonding0 comments share save hide report 100% Upvoted This thread is archived New comments cannot be



Hydrogen Halides As Acids
/hydrofluoric-acid-molecule-147216065-5a7b7d47ba61770036b929f4.jpg)



Is Hydrofluoric Acid Hf A Strong Or Weak Acid
Wouldn't the greater difference in electronegativity mean that the F would pull the electron density more strongly and make it more likely for the proton to "leave"?Hydroiodic acid HI IIodide 10 * 10 9 Hydrobromic acid HBr BrBromide 13 * 10 6 Hydrochloric acid HCl ClChloride 10 * 10 3 Sulfuric acid H 2 SO 4 HSO 4Hydrogen sulfate ion 24 * 10 1 Nitric acid HNO 3 NO 3Nitrate ionHydronium ion H 3 O H 2 O Water 54 * 102 Oxalic acid HO 2 C 2 O 2 H HO 2 C 2 O 2Hydrogen oxalate ion 13 * 102 Sulfurous acidHydrofluoric acid or HF is an extremely powerful, corrosive acid This makes HF the only hydrohalic acid that isn't classified as a strong acid (eg, HCl, HBr, HI) With this in view why HF is a weak acid than HCl?



Acidity Trends In Organic Chemistry Master Organic Chemistry



Arrange The Following Order Of Property Indicated Against Each Set I Hf Hcl Hbr Hi Increasing Youtube
An 01 M HF solution is moderately acidic Water is much less acidic, and the acidity of ammonia is so small that the chemistry of aqueous solutions of this compound is dominated by its ability to act as a base HF pH = 21 H 2 O pH = 7 NH 3 pH = 111 The Size of the X Atom At first glance, we might expect that HF, HCl, HBr, and HI would become weaker acids as we go down this



2
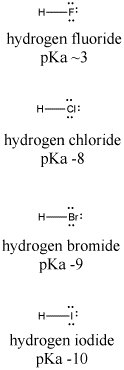



Ab11 Factors Affecting Bronsted Lowry Acidity



Why Hf Is The Weakest Acid Among Hcl Hbr And Hi Though Its Electronegetivity Is Highest Quora




Efficient Capture Of Actinides From Strong Acidic Solution By Hafnium Phosphonate Frameworks With Excellent Acid Resistance And Radiolytic Stability Sciencedirect




In Dilute Aqueous Solution Hf Is A Weaker Acid Than Hi Because




Acid Base Equilibria N K A And K B N Dissociation Of Weak Acid Bases N Ph Of Weak Acid Base Solutions N Ph Of Salt Solutions N Buffers N Ph Of Buffer



Why Is Hi The Strongest Acid Although It Has The Lowest Electronegativity Among Hydrogen Halides Quora



Which Acid Is More Acidic In Nature Hf Or Hi Quora




Crash Course In Which Of The Following Arrangements The Sequence Is Not Strictly According To The




Solved Hcl Hbr And Hi Are Strong Acids However Hf Is A Chegg Com



2



Acids Bases And Salts Chapters 14 And 15



Chapter 6 Reactions Of Alkenes Addition Reactions 6



1




Periodic Variations Of Acidic And Basic Properties Pdf Acid Dissociation Chemistry



Why Hf Is The Weakest Acid Among Hcl Hbr And Hi Though Its Electronegetivity Is Highest Quora




Pka Pka 10 Hi Among The Hydrogen Halides The Acidity Is Hi Hbr Hci Hf 5 Homeworklib




Chemistry The Central Science Chapter 16 Section 10




Horizons Hf Hm And Hs In Graphical Definition Download Scientific Diagram



2



Ab11 Factors Affecting Bronsted Lowry Acidity




Need Help On These 3 Acid Base Chemistry A 10 Points List The Pka Of Each Compound Homeworklib



5 Key Factors That Influence Acidity In Organic Chemistry



Acids And Bases Chapter 15 1 Copyright The



Part I Acids Bases N Several Concepts Of



1



1 15 Predicting Relative Acidity Chemistry Libretexts



Factors The Control The Relative Strengths Of Acids And Bases




Hi Is Stronger Than Hf In Acidic Strength Why Hf क त लन म Hi अध क अम ल य ह क य Youtube




The Boiling Points Of Hf Hcl Hbr And Hi Follow The Order



Organic Acids And Bases



Solved 17 Which Of The Following Is The Strongest Acid A Hf B Hbr C Hci D Hi 18 Which Of The Following Is The Strongest Course Hero
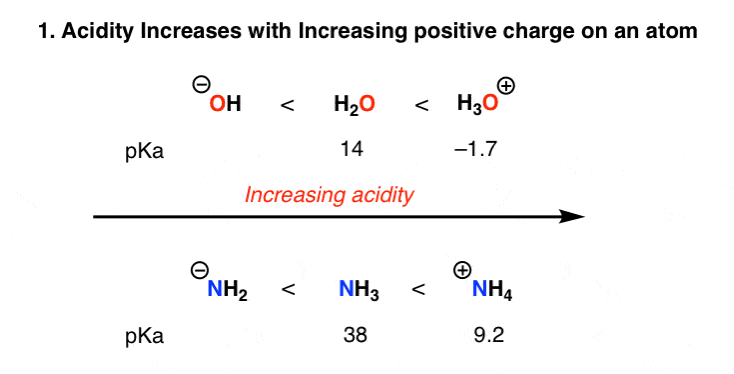



5 Key Factors That Influence Acidity In Organic Chemistry



Why Is Hf More Acidic Than Ch3cooh Quora




Consider The Following Orders I Hf Hcl Hbr Hi Acidic Strength Ii Ch 2 Lt Ccl 4 Gt Cf 4 Electronegativity Of Central C Atom Iii Mg 2 Lt K




Is Hf A Strong Acid Techiescientist




Rank In Order Of Increasing Acidity Hf Hcl Hbr Hi A Hf Hcl Hbr Hi B Hi Hbr Hcl Course Hero




Is Hydrofluoric Acid A Strong Or Weak Acid




Why Is Hf More Acidic Than Ch4 While An F Ion Is More Electronegative Quora
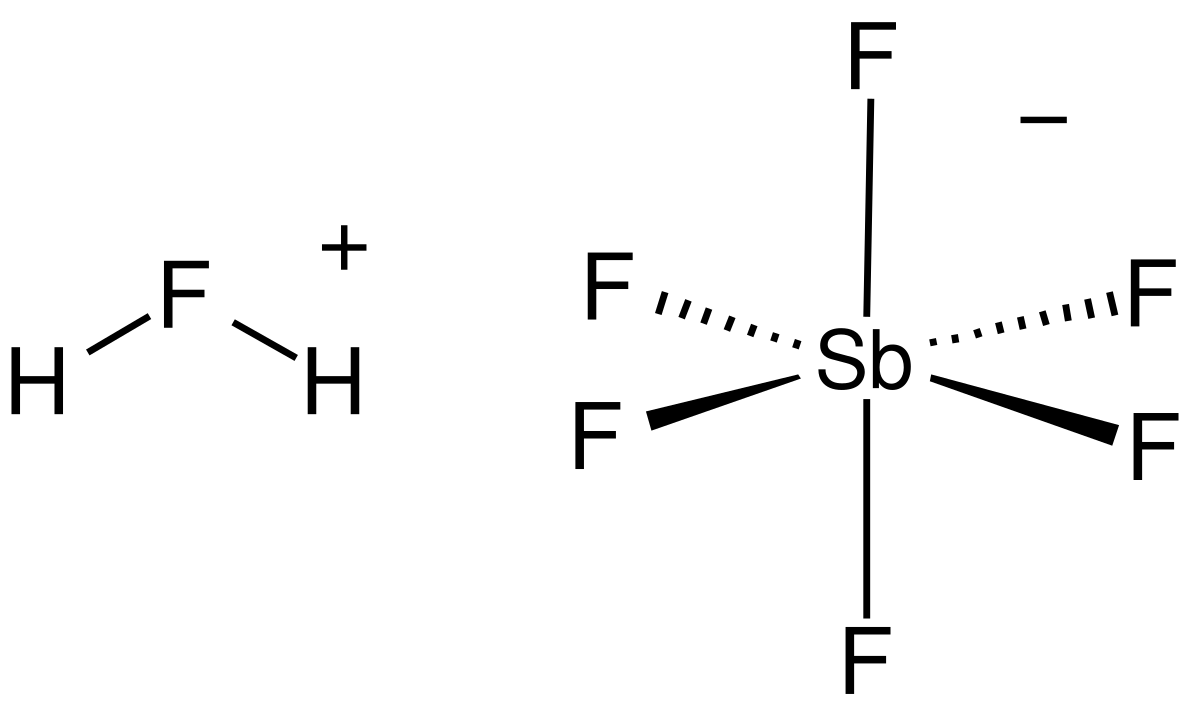



Fluoroantimonic Acid Wikipedia
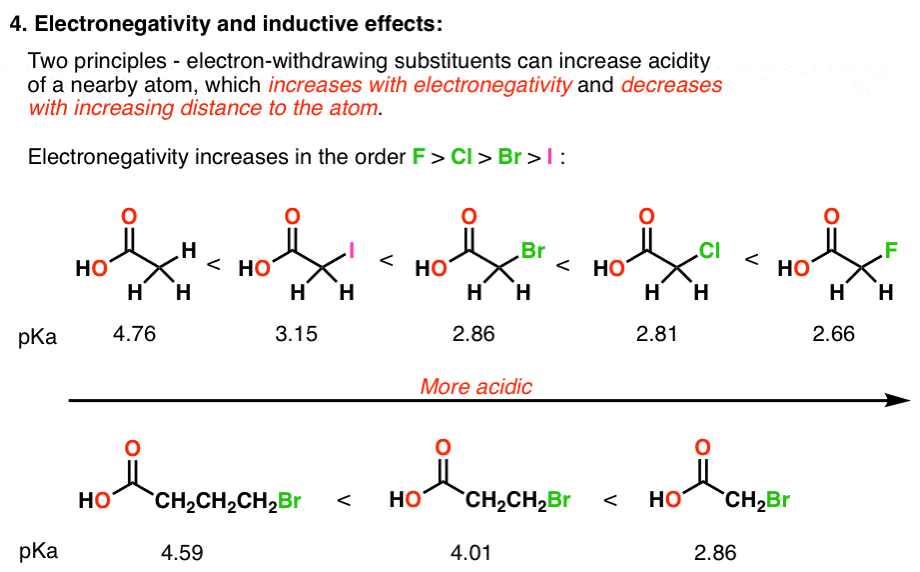



5 Key Factors That Influence Acidity In Organic Chemistry




Solved Question 31 Arrange These Acids In The Order Of Chegg Com




Rationalizing Strength Of Binary Halogen Acids With Mo Theory Chemistry Stack Exchange
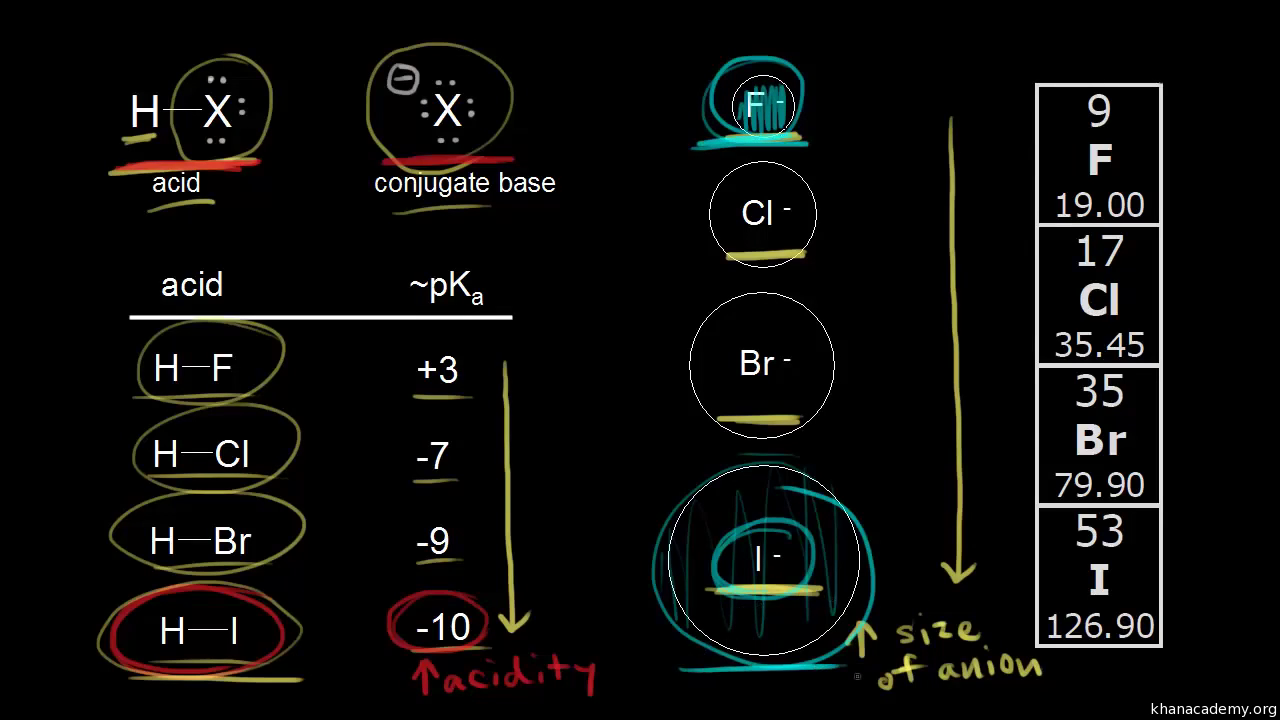



Acid Strength Anion Size And Bond Energy Video Khan Academy




Which Is The Strongest Acid Among These H Clutch Prep




Hi Is Stronger Than Hf In Acidic Strength Why




Solved 4 Rank The Compounds In Each Of The Following Groups Chegg Com




The Acidity But It Should Be Noted That While Comparing Elements In The Same Group Of
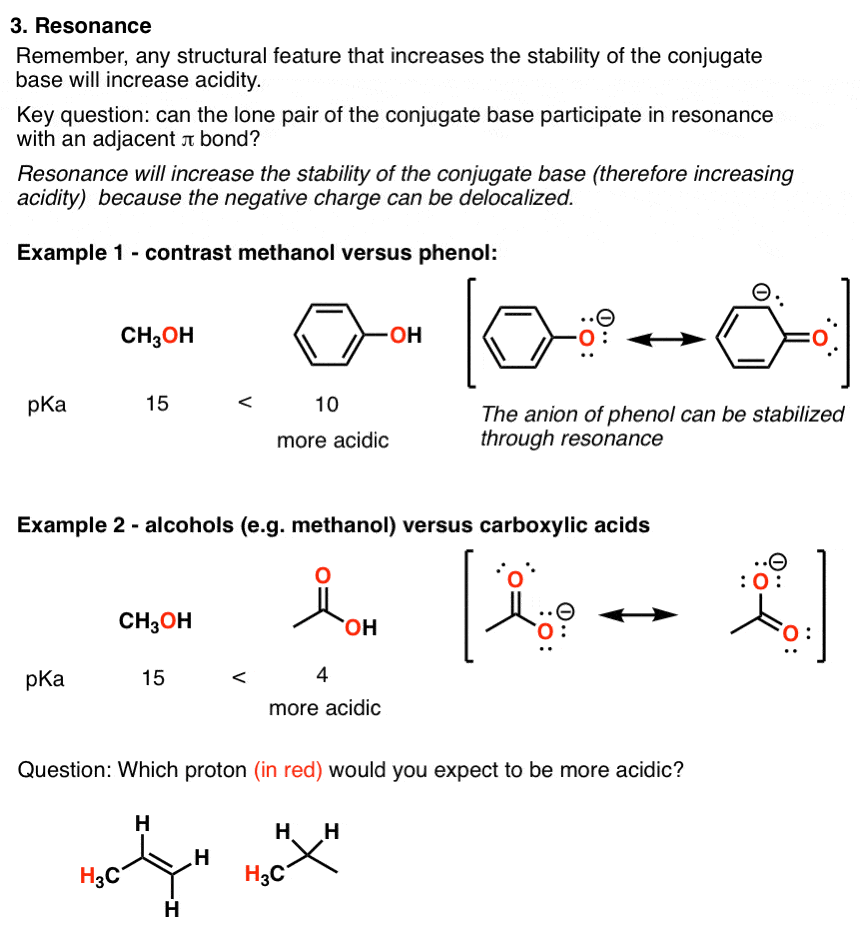



5 Key Factors That Influence Acidity In Organic Chemistry




Depth Integrated So4 A Fe B And Acidity C Production Download Scientific Diagram




Oxoacids And Acid Strength Pdf Chemical Polarity Acid



Hf




Which Hydrogen Halide Has The Strongest Bond Hf Hcl Hbr Hi Chemical Bonding Youtube Youtube
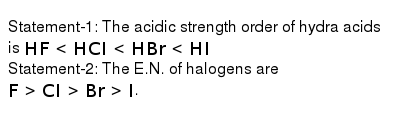



Statement 1 The Acidic Strength Order Of Hydra Acids Is Hf Lt Hci Lt Hbr Lt Hi Statement 2 The E N Of Halogens Are F Gt Ci Gt Br Gt I




Hi Is Stronger Than Hf In Acidic Strength Why




Solved 17 Which Of The Following Compounds Is Most Acidic Chegg Com



Why Hf Is The Weakest Acid Among Hcl Hbr And Hi Though Its Electronegetivity Is Highest Quora




Select The Correct Order Of Acidity A Hi Hbr Hci Hf B Hcio Hbro Hio C Hcio Hbro Hio D Hcio Hcio Hcio Hcio




A Account For The Following I Acidic Character Increase From Hf To Hi Ii There Is A Large Difference Between The Melting And Boiling Points Of Oxygen And Sulphur Iii Nitrogen Does Not Form Pentahalide B Draw The



Why Hf Is The Weakest Acid Among Hcl Hbr And Hi Though Its Electronegetivity Is Highest Quora




Down The Group Acidic Character Decreases Then Why In Halogen The Order Is Hi Hbr Hcl Hf Chemistry The P Block Elements Meritnation Com
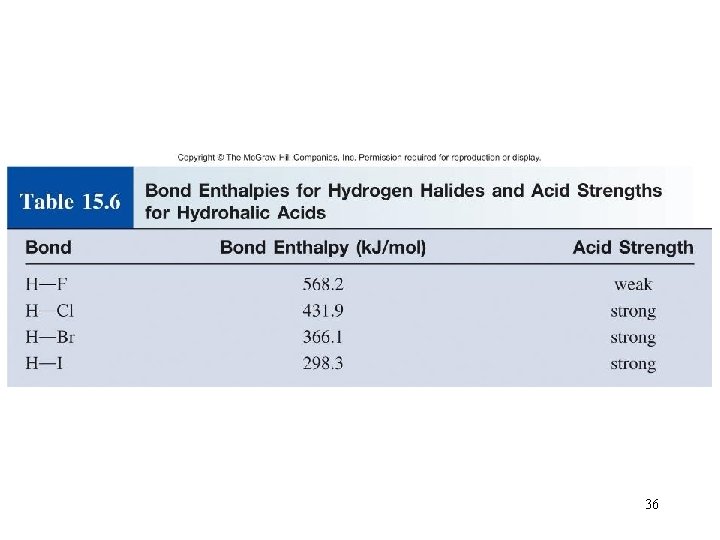



Acids And Bases Chapter 15 1 Copyright The




Big Picture Perspective Acid Base Chemistry Is Highly Diverse Encompassing Not Only The Traditional H Oh Chemistry That Characterizes Aqueous Solutions Ppt Download




Is Hf A Strong Acid Techiescientist




Why Hydrogen Fluoride Is A Weaker Acid Than Hydrochloric Acid Find Acidic Order Of Hf Hcl Hbr Hi Youtube




Rationalizing Strength Of Binary Halogen Acids With Mo Theory Chemistry Stack Exchange



Solved Klein 3 4 3 6 Ax Dawwin 1 What Are Four Factors That Chegg Com



2




Need Help On These 3 Acid Base Chemistry A 10 Points List The Pka Of Each Compound Homeworklib




Assertion A Increasing Order Of Acidity Of Hydrogen Halides Is Hf Lt Hcl Lt Hbr Lt Hi Reason R While Comparing Acids Formed By The Elements Belonging To The Same



1
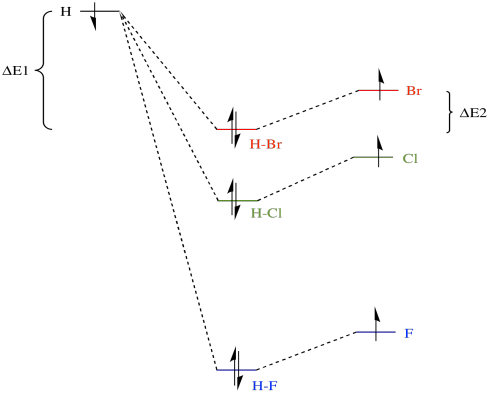



Ab11 Factors Affecting Bronsted Lowry Acidity



0 件のコメント:
コメントを投稿